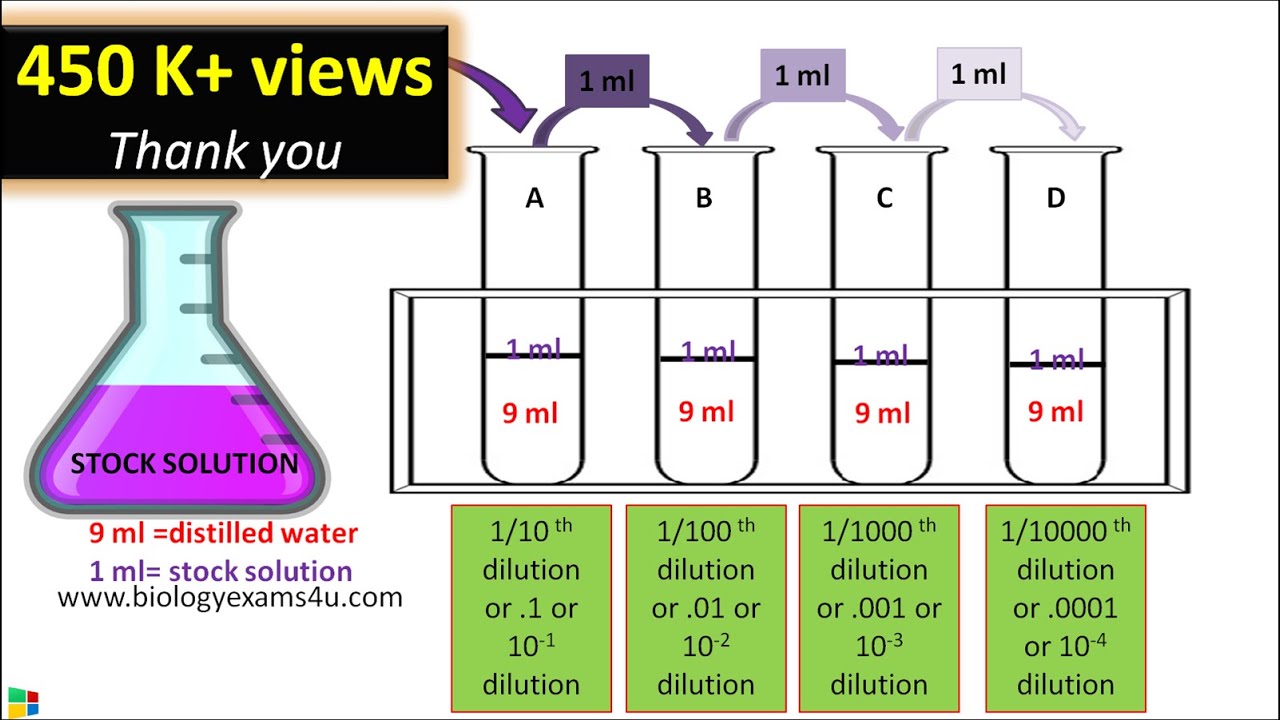
A dilution solution contains solute (or stock solution) and a solvent (called diluent). These two components proportionally combine to create a dilution. You can identify a dilution solution by the amount of solute in the total volume, expressed as a proportion. For example, a chemical may be prepared in a 1:10 dilution of alcohol, indicating that a 10 mL bottle contains one milliliter of chemical and nine milliliters of alcohol. You can calculate the necessary volume of each component to prepare a dilution solution. Download aplikasi pembobol wifi untuk pc world laptop.
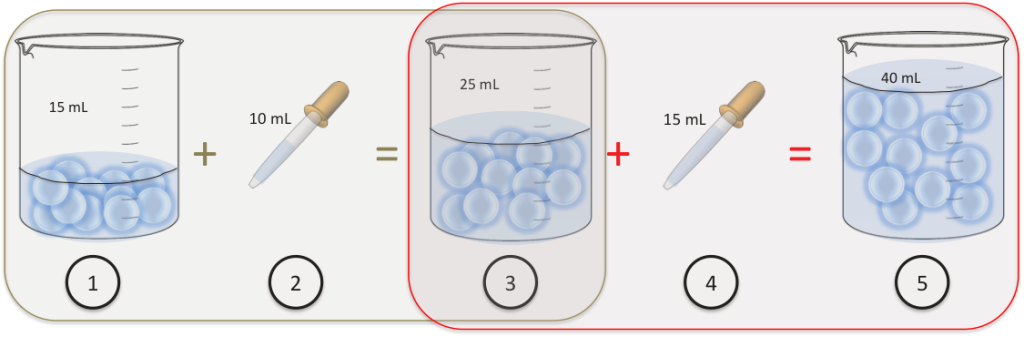
Feb 17, 2016 A very quick lesson on how to do the math for serial and simple dilutions. Skip navigation Sign in. Blanidas 300 metodicheskie ukazaniya po primeneniyu. Serial dilutions lesson Kim Wiest. Liters Volume Calculations Chemistry - Duration.